Sanitary valves comply with strict health and safety requirements in the food, beverage, and medical industries. The bodies of sanitary valves are built of stainless steel that complies with strict health regulations. Every component that comes into contact with a liquid, gas, or slurry medium has been mirror polished. They are used in hospitals, pharmaceutical companies, and food processing facilities.Read More…
It is our primary goal that we are able to treat each and every customer as if they were number one in hopes of turning first time customers into lifelong connections as well as to exceed customer expectation one hundred percent of the time in order to keep you coming back to us for all of your diaphragm valve needs! To receive more information about our company get in touch with our customer...

At Aquamatic, we take pride in our commitment to engineering excellence and innovation. Our expertise lies in the design and production of cutting-edge diaphragm valves that set industry standards. With a rich history of delivering top-notch solutions, we, at Aquamatic, are dedicated to providing unparalleled performance and reliability in fluid control.

Watson-Marlow Fluid Technology is a leader in the diaphragm valve industry. Our expert team works side by side with our customers to ensure that our products fit their needs. For more information about our diaphragm valves, visit our website or give us a call today!

We manufacture a variety of butterfly control valves—stainless steel, lug, wafer butterfly valves, standard, custom, shut-off and more. ITT knows the various chemical, fluid and gas processes and engineers products accordingly. Our brand names include Fabri-Valve®, Richter™, Pure-Flo®.

More Sanitary Diaphragm Valve Manufacturers
However, sanitary valves are also useful in settings with strict health regulations that forbid any tolerance for chemical and physical dangers.

Types of sanitary valves
Sanitary valves are categorized by how they mechanically move. For example, linear motion, rotary motion, and quarter-turn valves are three different classes of sanitary valves.
Linear motion valves enable, halt, or throttle flow using a closing member. Gate valves, globe valves, diaphragm valves, pinch valves, and lift check valves are standard linear motion valves.

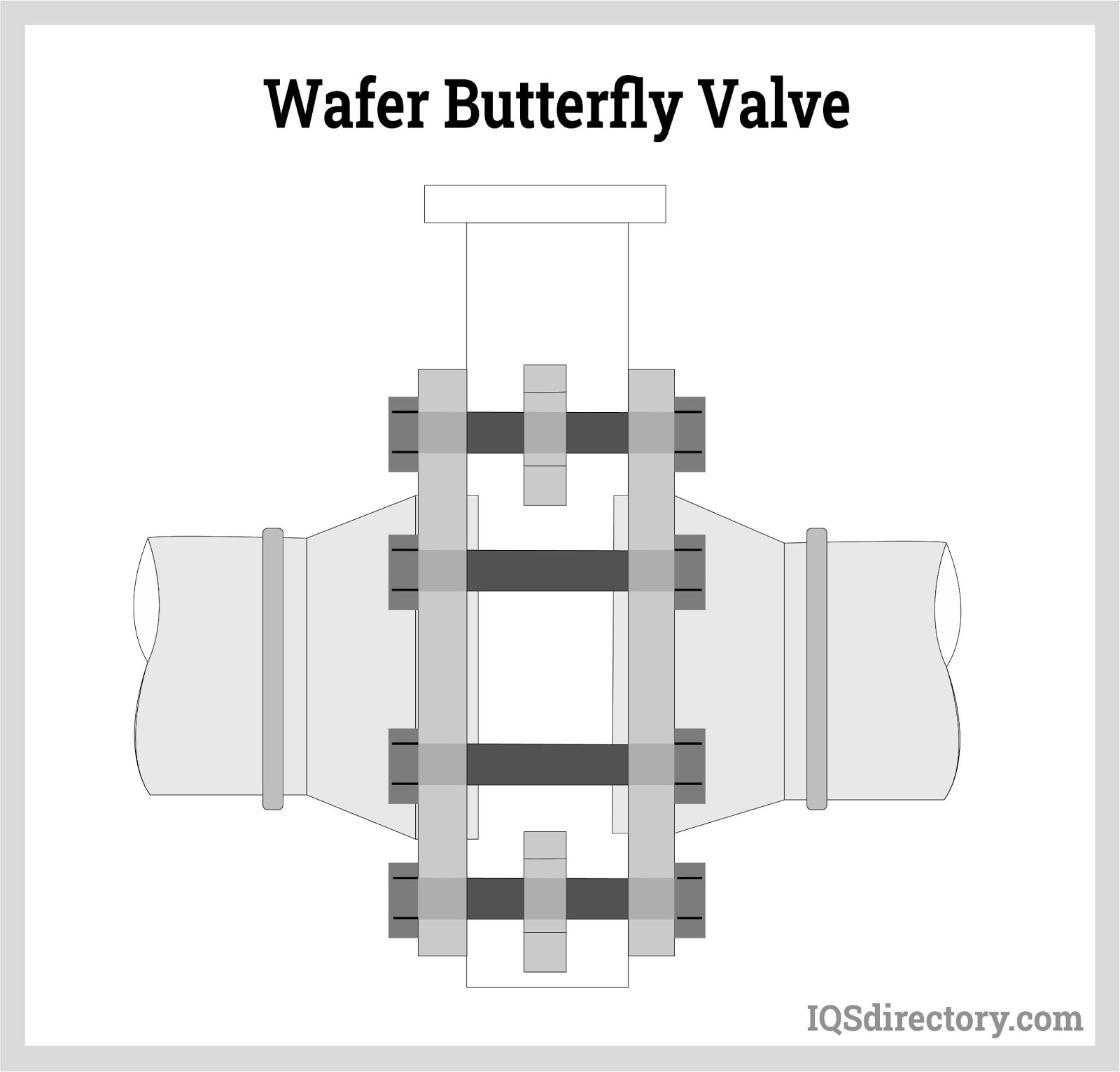
In rotary motion valves, the component that closes the valve travels in an angled or cylindrical route. Some common rotary motion valves are butterfly, ball, plug, eccentric, and swing check valves.
In essence, quarter-turn valves are rotational motion valves that may be turned from fully open to fully closed or vice versa with just a quarter-turn of the stem motion.
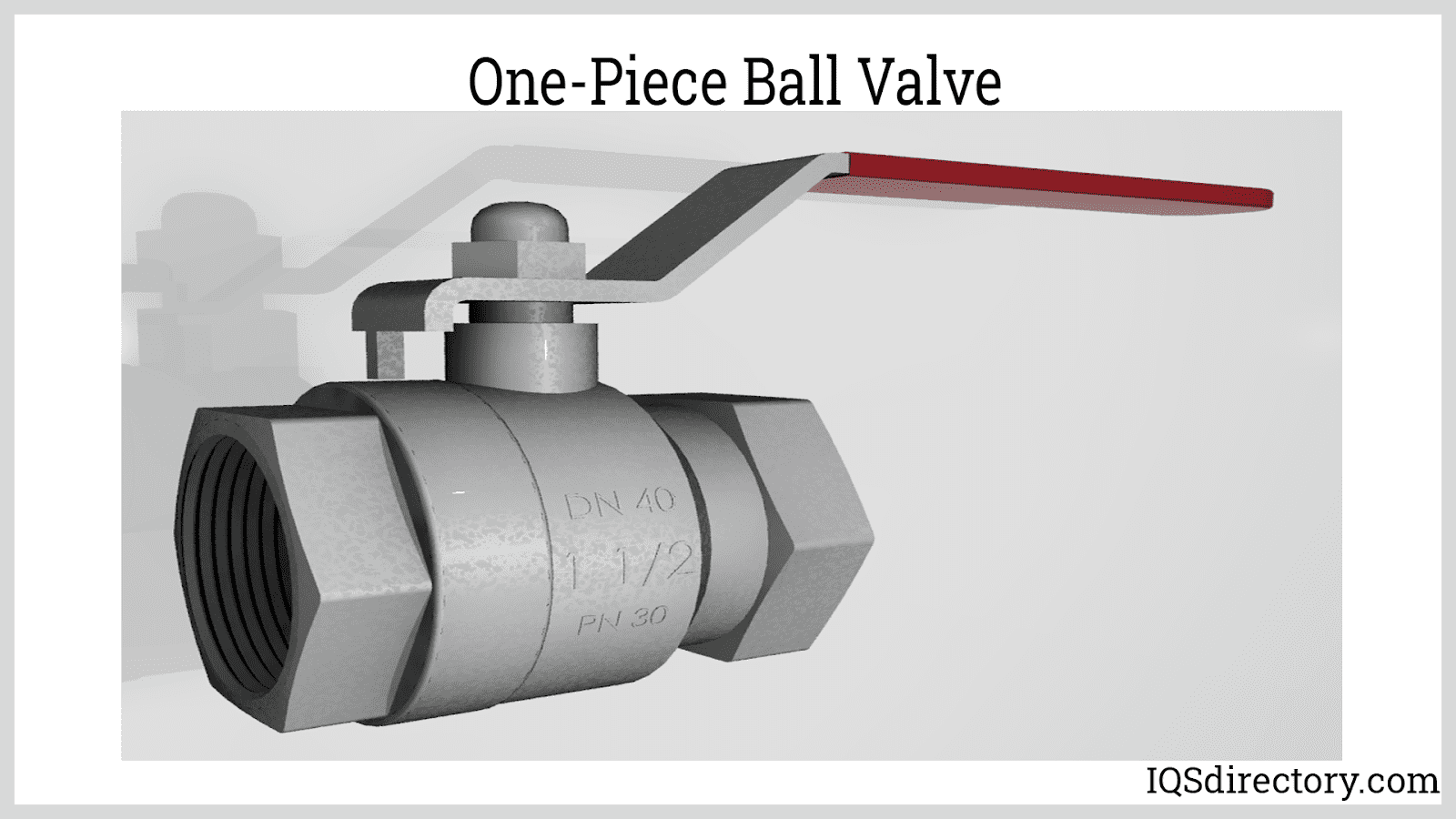
Remember that sanitary valves must adhere to unique sterility standards, particularly for the valve body and sealing elements. In addition, the valve seat's sealing surface must be non-toxic. Rubber and stainless steel are suitable materials for hard and soft sealing. The most common sanitary valve types are sanitary ball valves and sanitary butterfly valves. Sanitary check valves, solenoid valves, and diaphragm valves are also used throughout the industry.

Mechanical Sealing Requirements for Sanitary Valves
Sanitary valves must follow strict mechanical sealing specifications. The contact surface must be easy to clean and disinfect. Manufacturers must ensure a fluid transition and flow with no material buildup. Material flowing from the valve shouldn’t be able to escape from the sealing surface.
Applications of a Sanitary Valve
In the food and beverage sector, sanitary valves connect and regulate conveyance pipelines that process fluid and semi-fluid materials.
Sanitary valve material construction requirements vary depending on the type of industrial use. However, the most common material used is stainless steel.
Advantages of Sanitary Diaphragm Valves
- Sanitary valves have nylon sleeves to reduce friction and simplify the opening and closing process.
- Sanitary valves are made of food-grade rubber and stainless steel. They are useful for products that are susceptible to external contamination.
- Sanitary butterfly valve handles are resilient to torque from pulling or wrenching. As a result, they are less likely to fracture.
- Sanitary valves often have detachable connections for easy repair and cleaning.
- Sanitary valves must pass rigorous strength and sealing testing.
Disadvantages of Sanitary Diaphragm Valves
- Ball valves should not be used for continuous throttling.
- The particles of excess fluids adhere to the surface and could lead to leaks, abrasion, and other issues.
Choosing the Right Sanitary Valve Manufacturer
To ensure you have the most productive outcome when purchasing sanitary valves from a sanitary valve manufacturer, it is important to compare several companies using our directory of sanitary valve companies. Each sanitary valve manufacturer has a business profile page highlighting their experience and capabilities, along with a contact form to directly communicate with this manufacturer for more information or request a quote. Review each sanitary valve company using our patented website previewer to get an idea of what each business specializes in. Then, use our simple RFQ form to contact multiple sanitary valve companies with the same form.









 Ball Valves
Ball Valves Butterfly Valves
Butterfly Valves Centrifugal Pumps
Centrifugal Pumps Check Valves
Check Valves Diaphragm Valves
Diaphragm Valves Flow Meters
Flow Meters Hydraulic Pumps
Hydraulic Pumps Hydraulic Valves
Hydraulic Valves Metering Pumps
Metering Pumps Solenoid Valves
Solenoid Valves Vacuum Pumps
Vacuum Pumps Castings & Forgings
Castings & Forgings Bulk Material Handling
Bulk Material Handling Electrical & Electronic Components
Electrical & Electronic Components Flow Instrumentation
Flow Instrumentation Hardware
Hardware Material Handling Equipment
Material Handling Equipment Metal Cutting Services
Metal Cutting Services Metal Forming Services
Metal Forming Services Metal Suppliers
Metal Suppliers Motion Control Products
Motion Control Products Plant & Facility Equipment
Plant & Facility Equipment Plant & Facility Supplies
Plant & Facility Supplies Plastic Molding Processes
Plastic Molding Processes Pumps & Valves
Pumps & Valves Recycling Equipment
Recycling Equipment Rubber Products & Services
Rubber Products & Services